Abstract
Background: Pts with previously treated R/R aggressive LBCL have compromised health-related QOL (HRQOL). Liso-cel is an autologous, CD19-directed, defined composition, 4-1BB CAR T cell product administered at equal target doses of CD8 + and CD4 + CAR + T cells. In a prespecified interim analysis of TRANSFORM (NCT03575351), a randomized, open-label, pivotal trial, liso-cel demonstrated statistically significant and clinically meaningful improvement in the primary endpoint of event-free survival and key secondary endpoints (complete response rate and progression-free survival) in adults with R/R LBCL after failure of first-line (1L) immunochemotherapy compared with SOC, with no new safety signals. Here we present results of the pt-reported outcomes (PRO) analysis from TRANSFORM.
Methods: Adults (age ≤ 75 yrs) with R/R LBCL (≤ 12 mo after 1L therapy), who were eligible for autologous stem cell transplantation (ASCT), were randomized to receive either SOC (3 cycles of salvage chemotherapy [CT] and BEAM + ASCT for responding pts) or liso-cel after lymphodepletion. Crossover to receive liso-cel was allowed in the SOC arm for pts who failed treatment. The European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire - 30 items (EORTC QLQ-C30) and the Functional Assessment of Cancer Therapy - Lymphoma Subscale (FACT-LymS) were administered at randomization (baseline) and on Days 29 (infusion of liso-cel or 2 cycles of salvage CT), 64 (1 mo post liso-cel or completion of CT), 126 (3 mos post liso-cel or 2 mos post ASCT), and Mo 6 and other prespecified timepoints up to Mo 36 or end of study. No PRO data were collected after crossover. The analysis was based on the PRO-evaluable population (pts with a baseline and ≥ 1 post-baseline assessment). Predefined thresholds determined clinically meaningful changes. Global health/QOL (GH/QOL), physical functioning, cognitive functioning, fatigue, pain, and FACT-LymS were the primary domains of interest based on their relevance to the study population and treatment. A linear mixed model for repeated measures (MMRM) analysis was performed to assess the between-treatment difference in overall least squares (LS) mean change from baseline for each primary domain, using data collected up to Day 126 for visits with a sample size per arm ≥ 10. Proportions of pts with meaningful change from baseline were assessed for each primary domain up to Mo 6. All analyses were descriptive only.
Results: Of 184 randomized pts, 90 (49%) and 85 (46%), respectively, were included in the PRO-evaluable population for the EORTC QLQ-C30 (SOC vs liso-cel n=43 vs 47) and FACT-LymS (n=40 vs 45, respectively). The PRO assessment completion rate from baseline up to Mo 6 was ≥ 45%, which was lower than expected primarily due to operational challenges during the COVID-19 pandemic but was comparable for both arms.
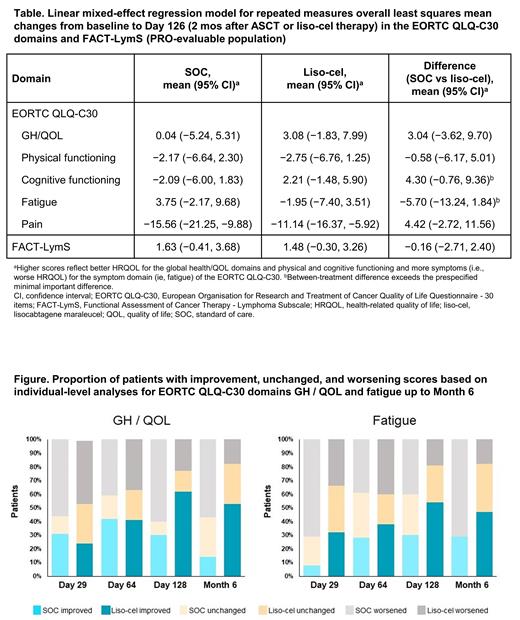
In the MMRM analysis, the liso-cel arm had more favorable overall LS mean changes from baseline to Day 126 than the SOC arm in most of the EORTC QLQ-C30 domains and FACT-LymS. In particular, the between-treatment differences for cognitive functioning (−2.09 vs 2.21) and fatigue (3.75 vs −1.95) for SOC versus liso-cel, respectively, exceeded the prespecified minimal important difference threshold (Table); in those domains, the SOC arm deteriorated while the liso-cel arm improved.
In individual-level analyses, the proportion of pts with meaningful improvement for fatigue and GH/QOL was higher, while deterioration was lower, in the liso-cel arm versus SOC arm from baseline up to Mo 6 (Figure). At Mo 6, a higher proportion of pts experienced worsened fatigue (71% vs 18%) and a lower proportion experienced improved fatigue (29% vs 47%) in the SOC arm compared with the liso-cel arm; for GH/QOL, a higher proportion of pts worsened (57% vs 18%) and lower proportion improved (14% vs 53%), respectively. For the other primary domains, the proportions of pts with improvement or deterioration favored liso-cel or were similar between arms.
Conclusions: Compared with SOC, liso-cel showed favorable improvement in most primary PRO domains, particularly EORTC QLQ-C30 cognitive functioning and fatigue and more pts showed PRO improvements and fewer showed deterioration by Mo 6 with liso-cel. The results were achieved despite only responders remaining in the SOC arm after salvage CT. HRQOL was either improved or maintained after liso-cel treatment in pts with R/R LBCL after failure of 1L therapy.
Abramson: Bristol-Myers Squibb Company: Consultancy, Research Funding; Morphosys: Consultancy; C4 Therapeutics: Consultancy; Kite Pharma: Consultancy; Kymera: Consultancy; Incyte Corporation: Consultancy; Bluebird Bio: Consultancy; Astra-Zeneca: Consultancy; Allogene Therapeutics: Consultancy; Novartis: Consultancy; EMD Serono: Consultancy; Genmab: Consultancy; Seagen Inc.: Research Funding; AbbVie: Consultancy; Karyopharm: Consultancy; Genentech: Consultancy; BeiGene: Consultancy. Arnason: Juno/BMS: Honoraria. Glass: BMS: Consultancy; Roche: Consultancy, Research Funding, Speakers Bureau; Riemser: Research Funding; Kite: Consultancy; Novartis: Consultancy; Helios Klinik Berlin-Buch: Current Employment. Crotta: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Montheard: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Previtali: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Liu: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Braverman: BMS: Current Employment, Current equity holder in publicly-traded company. Guo: Daiichi Sankyo: Consultancy; UCB: Consultancy; Janssen: Consultancy; Gilead: Consultancy; Bristol Myers Squibb: Consultancy; EMD Serono: Consultancy; Evidera: Current Employment. Shi: Bristol Myers Squibb: Consultancy. Kamdar: ADC Therapeutics: Consultancy; Adaptive Biotechnologies: Consultancy; TG Therapeutics: Research Funding; Genentech: Research Funding; AbbVie: Consultancy; KaryoPharm: Consultancy; Kite: Consultancy; AstraZeneca: Consultancy; SeaGen: Speakers Bureau; Celgene: Other; Genetech: Other; Celgene (BMS): Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal